
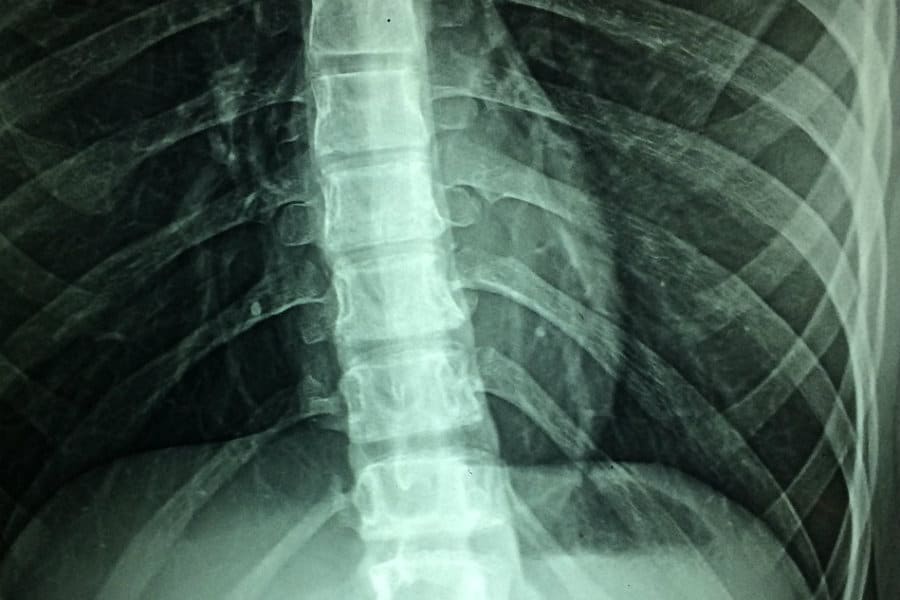

Moreover, adverse neurologic effects of this therapy are not well known or understood. The precise mechanism of spinal cord stimulation has not been fully elucidated. The first-in-class high frequency spinal cord stimulator to gain FDA approval was the Senza HF10 device from Nevro Corporation (Redwood City, CA). Specifically, high frequency stimulation relies on a continuous waveform delivered at 10 kHz. These include burst stimulation, high pulse density, and high frequency stimulation. Recently, new classes of “paresthesia-free” waveforms have demonstrated long-term durable pain relief. Spinal cord stimulator technology until 2015 had relied on low frequency electrical stimulation in the 50-120 Hz range with either constant-current or constant-voltage waveforms to achieve paresthesia for pain relief. Since then, the technology of spinal cord stimulation has advanced at an accelerated pace. using a single epidural lead programmed at 10-50 Hz tonic frequency. The first clinical report of successful use of spinal cord stimulation (SCS) was described in 1967 by Shealy et al. Spinal cord stimulation is a proven treatment modality for a variety of pain pathologies. Further research and investigation in this area are needed so that clinicians and patients may have more complete knowledge and understanding of the potential treatment-limiting complications of spinal cord stimulation. Food and Drug Administration Manufacturer and User Facility Device Experience database are included. To further explore these less common neurologic complications of SCS therapy, a review of literature and a review of the U.S. These symptoms resolved following deactivation of her device. We present the case of a patient who underwent placement of a Senza HF10 high-frequency spinal cord stimulator with subsequent development of tinnitus, vertigo, intermittent involuntary left facial twitches, and perioral numbness. Less well understood are the adverse neurological effects of this therapy. The most common complications of spinal cord stimulation (SCS) therapy are generally related to surgical site infection and hardware malfunction.


 0 kommentar(er)
0 kommentar(er)
